Nursing Paper Example on Cold Agglutinin Disease
Nursing Paper Example on Cold Agglutinin Disease
Cold agglutinin disease is a rare autoimmune hemolytic anemia characterized by the premature destruction of red blood cells due to the activity of cold agglutinins. These are autoantibodies that become activated at low temperatures, typically below 37°C (98.6°F). The condition is often associated with underlying infections, lymphoproliferative disorders, or may occur idiopathically. Patients with cold agglutinin disease experience a range of symptoms, including fatigue, hemolysis-related anemia, and cold-induced symptoms such as acrocyanosis. Early diagnosis and tailored management strategies are essential to improve patient outcomes.
Causes of Cold Agglutinin Disease
Primary Cold Agglutinin Disease
This idiopathic form has no identifiable underlying cause and accounts for most cases in older adults.
Secondary Cold Agglutinin Disease
This form is associated with other conditions, including:
Infections: Mycoplasma pneumoniae, Epstein-Barr virus, and cytomegalovirus infections are common triggers.
Lymphoproliferative Disorders: Conditions such as Waldenström macroglobulinemia or non-Hodgkin lymphoma often result in cold agglutinin production.
Autoimmune Diseases: Rarely linked to systemic lupus erythematosus or other autoimmune conditions.
Genetic and Environmental Factors
While not directly inherited, certain genetic predispositions may influence immune system dysregulation, increasing susceptibility.
Signs and Symptoms
Hemolysis-Related Symptoms
Fatigue and pallor due to anemia.
Jaundice from elevated bilirubin levels caused by red blood cell breakdown.
Dark urine from hemoglobinuria.
Cold-Induced Symptoms
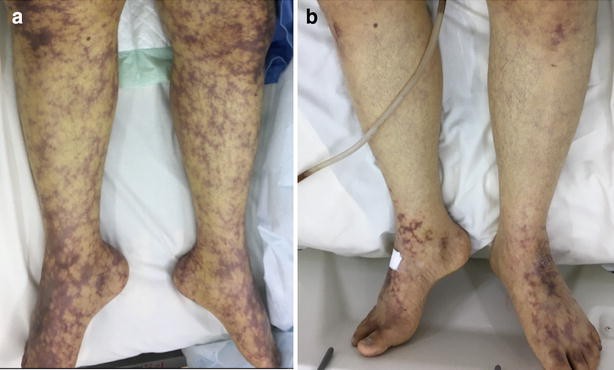
Acrocyanosis: Bluish discoloration of extremities, especially fingers, toes, and ears, due to red blood cell agglutination.
Raynaud’s phenomenon: Cold-induced discoloration and pain in the fingers and toes.
General Symptoms
Weakness and dizziness.
Splenomegaly, though less common than in other hemolytic anemias.
Etiology
Cold agglutinin disease results from the production of cold-reactive immunoglobulins, particularly IgM autoantibodies, that target red blood cell antigens. These antibodies activate the complement system, leading to intravascular and extravascular hemolysis.
Secondary forms are frequently associated with:
Infections: Mycoplasma pneumoniae induces transient cold agglutinin production during infection.
Lymphoproliferative Disorders: Abnormal B-cell activity contributes to chronic antibody production.
Pathophysiology
The disease mechanism involves:
Cold Agglutinin Activation
Autoantibodies bind to red blood cells in cooler parts of the body, such as the extremities. This agglutination clogs microvasculature and causes hemolysis.
Complement Activation
Cold agglutinins activate the complement cascade, particularly C3b deposition, leading to:
Intravascular Hemolysis: Direct red blood cell destruction in circulation.
Extravascular Hemolysis: Phagocytosis of complement-coated red blood cells in the liver.
Temperature Sensitivity
The antibodies become inactive at higher core body temperatures, confining hemolysis to areas exposed to cold.
Diagnosis
Clinical History and Physical Examination
Documenting cold sensitivity and hemolysis-related symptoms.
Identifying triggers such as infections or cold exposure.
Laboratory Tests
Complete Blood Count: Anemia with elevated reticulocyte count.
Peripheral Blood Smear: Evidence of agglutinated red blood cells.
Cold Agglutinin Titer: Confirmatory test showing high titers of cold-reactive IgM antibodies.
Complement Testing: Reduced C3 and C4 levels indicate complement activation.
Direct Antiglobulin Test: Positive for complement components but not for IgG.
Imaging and Further Testing
Chest X-ray: Rule out underlying Mycoplasma pneumoniae infection.
Bone Marrow Biopsy: Assess for lymphoproliferative disorders in unexplained cases.
Treatment Regimens
General Management
Avoiding Cold Exposure: Critical for reducing symptoms and preventing hemolysis.
Transfusions: Used for severe anemia but must be warmed to body temperature.
Pharmacological Treatments
Rituximab: First-line treatment targeting B-cells producing cold agglutinins.
Corticosteroids: Less effective but occasionally used to reduce inflammation.
Immunosuppressive Therapy: Agents like cyclophosphamide or bortezomib for refractory cases.
Treatment for Underlying Conditions
Infections: Antibiotic or antiviral therapy for secondary cold agglutinin disease caused by Mycoplasma pneumoniae or Epstein-Barr virus.
Lymphoproliferative Disorders: Chemotherapy or targeted therapies for conditions such as Waldenström macroglobulinemia.
Patient Education
Self-Management
Dress warmly, especially in cold climates, to reduce cold exposure.
Use gloves and other protective clothing to avoid exacerbation of symptoms.
Symptom Monitoring
Patients should be vigilant for worsening anemia symptoms, such as increased fatigue or jaundice.
Vaccinations
Routine influenza and pneumococcal vaccinations to prevent infections that may exacerbate symptoms.
Regular Follow-Up
Patients need ongoing evaluation to monitor disease progression and treatment response.
Additional Considerations
Prognosis
Primary cold agglutinin disease has a generally favorable prognosis with appropriate management.
Secondary forms improve with treatment of the underlying cause.
Complications
Severe anemia requiring repeated transfusions.
Thrombosis due to agglutinated red blood cells impairing blood flow.
Future Research
Advancements in targeted therapies, such as complement inhibitors, show promise in improving outcomes for patients with refractory disease.
Conclusion
Cold agglutinin disease is a rare but manageable autoimmune condition characterized by cold-induced hemolysis. Early diagnosis, avoidance of cold exposure, and targeted therapies, such as rituximab, significantly improve patient outcomes. Ongoing research and tailored patient education remain essential to further enhance quality of life and reduce disease burden.
References
Berentsen, S., & Sundic, T. (2015). Red blood cell destruction in autoimmune hemolytic anemia: Role of complement and potential new targets for therapy. Frontiers in Immunology, 6(395), 1-10. https://www.frontiersin.org/articles/10.3389/fimmu.2015.00395/full
Brodsky, R. A. (2021). Warm and cold autoimmune hemolytic anemias. New England Journal of Medicine, 384(15), 1401-1414. https://www.nejm.org/doi/full/10.1056/NEJMra2033980
Swiecicki, P. L., Hegerova, L. T., & Gertz, M. A. (2013). Cold agglutinin disease. Blood, 122(7), 1114-1121. https://doi.org/10.1182/blood-2013-02-453217
Harvard Health Publishing. (2023). Cold agglutinin disease. https://www.health.harvard.edu/a_to_z/cold-agglutinin-disease-a-to-z
American Society of Hematology. (2023). Cold agglutinin disease overview. https://www.hematology.org/education/patients/anemia/cold-agglutinin-disease



