Nursing Paper Example on Focal Segmental Glomerulosclerosis
Nursing Paper Example on Focal Segmental Glomerulosclerosis
Focal segmental glomerulosclerosis (FSGS) is a kidney disorder characterized by scarring in some parts of the glomeruli, the filtration units of the kidneys. It is a significant cause of nephrotic syndrome in children and adults and a leading cause of end-stage renal disease (ESRD). The disease is classified as primary, secondary, or genetic, with diverse etiologies and clinical manifestations. Understanding its pathophysiology, diagnostic criteria, and treatment options is crucial for effective management and improving patient outcomes.
Causes
The causes of FSGS are broadly categorized into primary, secondary, and genetic forms. Primary FSGS occurs without an identifiable cause and is often associated with circulating permeability factors that damage the glomerular filtration barrier. Secondary FSGS arises from identifiable conditions, such as obesity, infections like HIV, drug toxicity (e.g., heroin or pamidronate), and adaptive responses to kidney damage or hyperfiltration. Genetic FSGS involves mutations in genes encoding podocyte or glomerular basement membrane proteins, leading to structural abnormalities and impaired kidney function. Understanding the underlying cause is essential for determining appropriate management strategies.
Signs and Symptoms
FSGS presents with a spectrum of clinical features depending on its type and severity. Common symptoms include proteinuria (high levels of protein in the urine), which may be nephrotic (greater than 3.5 grams per day) or non-nephrotic. Patients may also experience edema (swelling), particularly in the legs, ankles, and face, due to hypoalbuminemia from protein loss. Hypertension is frequently observed in FSGS, and some patients may develop hematuria (blood in the urine). Over time, progressive kidney damage can lead to reduced glomerular filtration rate (GFR) and chronic kidney disease (CKD), culminating in ESRD if left untreated.
Etiology
The etiology of FSGS is diverse, encompassing immune-mediated, genetic, and environmental factors. Primary FSGS is believed to result from circulating permeability factors that disrupt podocyte function, although the exact nature of these factors remains elusive. Genetic forms of FSGS are associated with mutations in genes such as NPHS1, NPHS2, and ACTN4, which affect podocyte structure and function. Secondary FSGS results from conditions causing glomerular hyperfiltration or direct injury, such as reflux nephropathy, sickle cell anemia, or chronic drug use. Identifying the etiology is critical, as it informs treatment decisions and prognosis.
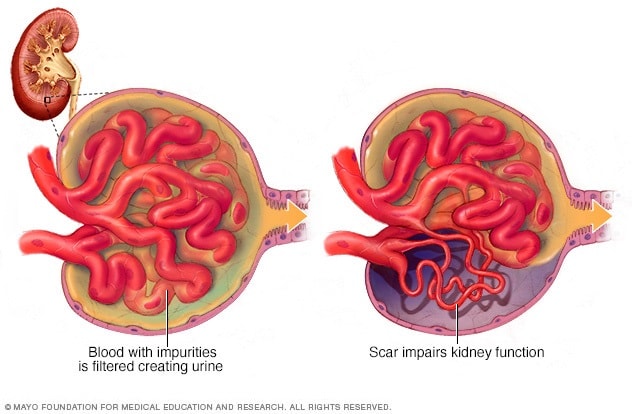
Pathophysiology
The hallmark of FSGS is podocyte injury, leading to structural and functional abnormalities in the glomeruli. Podocytes, essential for maintaining the glomerular filtration barrier, are particularly vulnerable to injury from immune factors, mechanical stress, or genetic mutations. Once podocytes are damaged, they detach from the glomerular basement membrane, causing glomerulosclerosis (scarring). This process reduces the kidney’s ability to filter waste and maintain fluid balance. Over time, compensatory hyperfiltration in unaffected glomeruli exacerbates the damage, creating a vicious cycle that accelerates disease progression and contributes to CKD.
Diagnosis
Diagnosing FSGS involves clinical evaluation, laboratory testing, and kidney biopsy. Proteinuria, a defining feature, is assessed through a 24-hour urine protein test or spot urine protein-to-creatinine ratio. Blood tests may reveal hypoalbuminemia, hyperlipidemia, and elevated creatinine levels, indicating impaired kidney function. Imaging studies like ultrasound are useful for assessing kidney size and structure. A definitive diagnosis requires kidney biopsy, which identifies segmental scarring in some glomeruli. Immunofluorescence and electron microscopy can further classify FSGS subtypes and help differentiate it from other glomerular diseases.
Treatment Regimens
Treatment for FSGS aims to reduce proteinuria, slow disease progression, and address the underlying cause. Primary FSGS is typically managed with immunosuppressive therapy, including corticosteroids, calcineurin inhibitors (e.g., tacrolimus, cyclosporine), and rituximab. Secondary FSGS requires addressing the underlying condition, such as treating obesity, controlling hypertension, or discontinuing nephrotoxic drugs. Renin-angiotensin-aldosterone system (RAAS) blockers, such as angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), are used to reduce proteinuria and preserve kidney function. In genetic FSGS, specific treatments depend on the genetic mutation involved, with supportive care playing a central role. Advanced cases may require dialysis or kidney transplantation, though recurrence in transplanted kidneys is a concern in primary FSGS.
Patient Education
Educating patients about FSGS is critical for effective disease management and improving quality of life. Patients should understand the importance of adhering to prescribed medications, dietary modifications (e.g., low-sodium and protein-restricted diets), and regular follow-up appointments to monitor kidney function. Lifestyle changes, such as maintaining a healthy weight and controlling blood pressure, are essential for slowing disease progression. For patients with secondary FSGS, addressing the underlying cause, such as reducing drug use or managing diabetes, is vital. Patients should also be aware of potential complications, including CKD and ESRD, and the need for timely intervention to prevent these outcomes.
Research and Future Directions
Current research on FSGS focuses on identifying the circulating factors implicated in primary FSGS, developing targeted therapies, and understanding the genetic basis of the disease. Advances in podocyte biology have provided insights into the mechanisms of podocyte injury and repair, opening new avenues for therapeutic interventions. Clinical trials are evaluating novel agents, such as endothelin receptor antagonists and novel immunomodulators, for reducing proteinuria and preserving kidney function. Precision medicine approaches, including genetic testing, are expected to improve the diagnosis and treatment of FSGS, offering personalized care for affected individuals.
Conclusion
Focal segmental glomerulosclerosis is a complex kidney disorder with diverse causes, manifestations, and outcomes. Early recognition, accurate diagnosis, and tailored treatment strategies are crucial for mitigating disease progression and preventing complications. Advances in understanding the pathophysiology of FSGS and ongoing research into novel therapies hold promise for improving outcomes for patients with this challenging condition. Patient education and lifestyle modifications remain integral components of comprehensive care, ensuring better disease management and quality of life.
References
D’Agati, V. D., & Kaskel, F. J. (2023). Focal segmental glomerulosclerosis: Pathophysiology and clinical features. Journal of the American Society of Nephrology. https://jasn.asnjournals.org
Chung, E. Y., & Wei, R. (2023). Current treatment approaches for FSGS. Clinical Journal of the American Society of Nephrology. https://cjasn.asnjournals.org
López-Gómez, J. M., & Morales, E. (2023). Genetic insights into focal segmental glomerulosclerosis. Kidney International. https://kidney-international.org
Rosenberg, A. Z., & Kopp, J. B. (2023). FSGS: Advances in diagnosis and therapy. Nature Reviews Nephrology. https://nature.com/nrneph
Meyrier, A. (2023). Therapeutic advances in primary and secondary FSGS. Nephrology Dialysis Transplantation. https://academic.oup.com/ndt