Nursing Paper Example on Candidiasis
Nursing Paper Example on Candidiasis
Causes
Signs and Symptoms
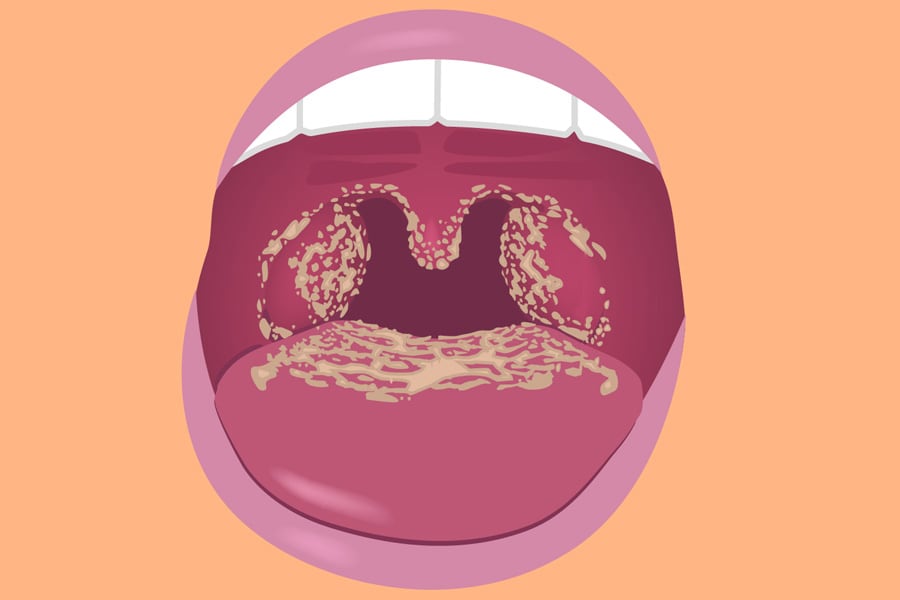
Candidiasis manifests differently depending on the affected area of the body. Common signs and symptoms include itching, redness, irritation, and discharge. In oral candidiasis, also known as thrush, white patches may appear on the tongue and inner cheeks. These patches can be painful and may bleed when scraped. Patients with oral thrush may also experience difficulty swallowing or altered taste sensations.
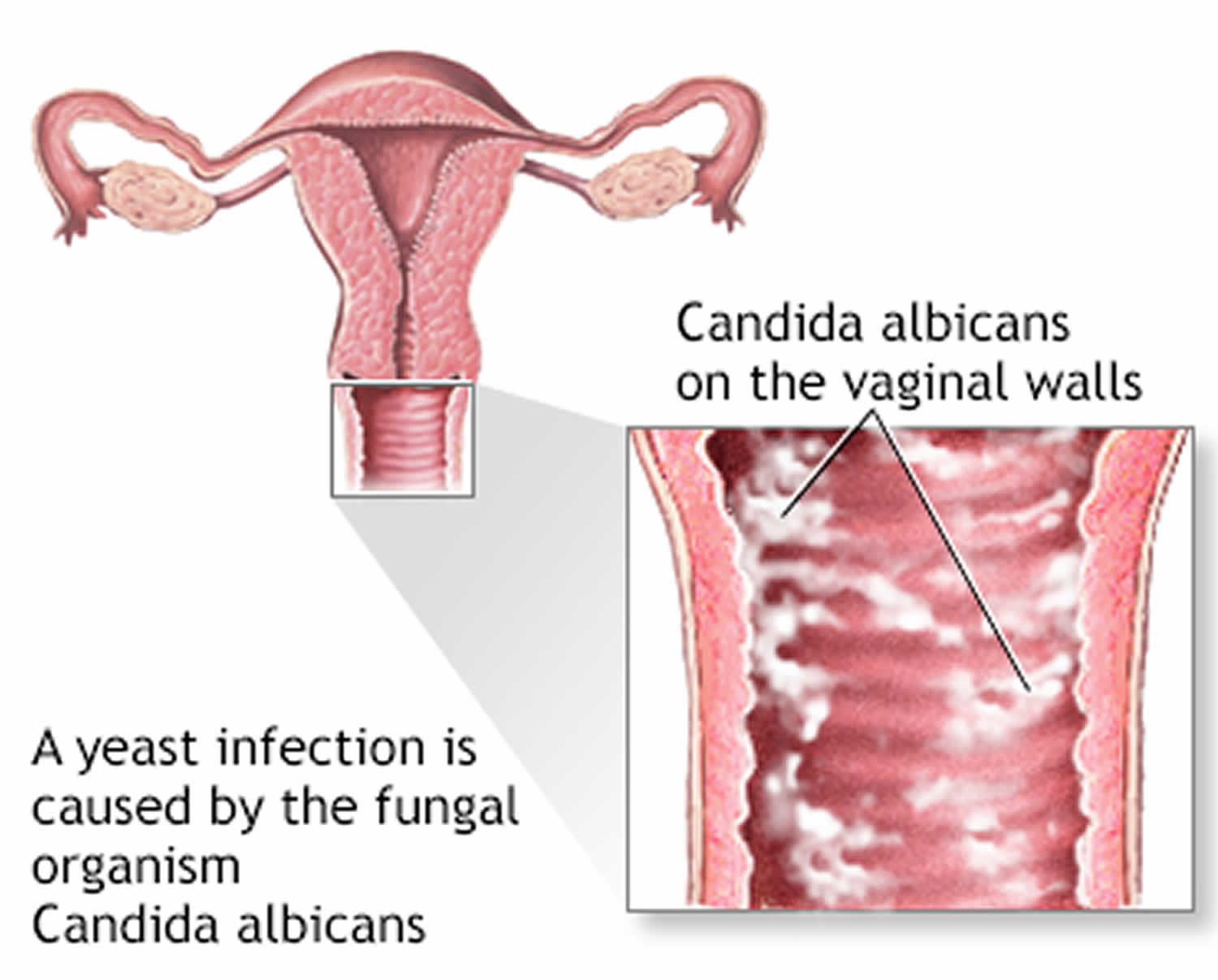
Vaginal candidiasis often presents with itching, burning, and abnormal discharge. The discharge is typically white, thick, and odorless, resembling cottage cheese. Itching and burning sensations may worsen during urination or sexual intercourse. In severe cases, vaginal candidiasis can lead to swelling and redness of the vulva, as well as fissures or cracks in the skin.
In cutaneous candidiasis, which affects the skin, red, itchy rashes with raised edges may develop in moist areas such as the groin, armpits, and under the breasts. These rashes can spread and become more inflamed if left untreated. In some cases, blisters or pustules may form, causing further discomfort.
For individuals with invasive candidiasis, symptoms may include fever and chills that do not improve with antibiotic treatment. Patients may also experience sepsis, a potentially life-threatening condition characterized by systemic inflammation and organ dysfunction. Invasive candidiasis often occurs in individuals with compromised immune systems, such as those with HIV/AIDS, cancer, or undergoing immunosuppressive therapy.
Additionally, candidiasis can affect the gastrointestinal tract, leading to symptoms such as abdominal pain, bloating, diarrhea, or constipation. These symptoms may be accompanied by oral thrush or genital candidiasis in some cases. Recognizing the signs and symptoms of candidiasis is crucial for prompt diagnosis and treatment to prevent complications and improve patient outcomes. (Nursing Paper Example on Candidiasis)
Etiology
The etiology of candidiasis is multifactorial, involving various predisposing factors that contribute to the overgrowth of Candida species. One of the primary factors is a weakened immune system, which diminishes the body’s ability to control the growth of opportunistic pathogens like Candida. Conditions that compromise immune function, such as HIV/AIDS, cancer, or autoimmune disorders, increase the susceptibility to candidiasis.
Prolonged or frequent use of antibiotics is another significant risk factor for candidiasis. Antibiotics disrupt the balance of microorganisms in the body, including beneficial bacteria that normally keep Candida in check. This disruption creates an environment conducive to Candida overgrowth, leading to infection.
Hormonal changes also play a role in predisposing individuals to candidiasis. Fluctuations in hormone levels, particularly estrogen, can affect the pH balance of the vagina, making it more susceptible to yeast overgrowth. This is commonly observed in pregnant women, women taking hormonal contraceptives, or those undergoing hormone replacement therapy.
Patients with diabetes mellitus are at increased risk of candidiasis due to elevated levels of glucose in bodily fluids. Candida species thrive in environments with high sugar content, such as the bloodstream and mucosal surfaces, leading to recurrent infections in diabetic individuals.
Poor hygiene practices, especially in moist and warm areas of the body, create an ideal environment for Candida overgrowth. Individuals who fail to maintain proper hygiene, such as washing and drying genital areas thoroughly, are more susceptible to genital candidiasis.
Furthermore, lifestyle factors such as wearing tight-fitting clothing, using irritating personal hygiene products, or having a diet high in sugar and refined carbohydrates can contribute to the development of candidiasis. By understanding the etiological factors associated with candidiasis, healthcare professionals can implement targeted interventions to reduce the risk of infection and improve patient outcomes. (Nursing Paper Example on Candidiasis)
Pathophysiology
DMS-5 Diagnosis
Diagnosing candidiasis involves a comprehensive assessment of clinical presentation, medical history, and, in some cases, laboratory tests to confirm the presence of Candida species. The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), does not provide specific diagnostic criteria for candidiasis; however, healthcare providers rely on clinical judgment and established guidelines to make accurate diagnoses.
Clinical presentation is a key component of candidiasis diagnosis, with symptoms varying depending on the affected area of the body. In vaginal candidiasis, patients typically report itching, burning, and abnormal vaginal discharge. Oral candidiasis presents with white patches on the tongue and inner cheeks, often accompanied by discomfort or difficulty swallowing. Cutaneous candidiasis may manifest as red, itchy rashes in skin folds or moist areas of the body.
Medical history is also essential in the diagnostic process, as certain predisposing factors increase the likelihood of candidiasis. Patients with a history of immunosuppression, diabetes mellitus, antibiotic use, or recent hospitalization are at higher risk for candidiasis and should be thoroughly evaluated.
Laboratory tests, while not always necessary, may be performed to confirm the diagnosis of candidiasis. Microscopic examination of vaginal or oral swabs can reveal the presence of yeast cells or hyphae, confirming the diagnosis of candidiasis. Additionally, culture-based methods may be employed to identify the specific species of Candida present and assess antifungal susceptibility in cases of recurrent or refractory infections.
Overall, diagnosing candidiasis requires a comprehensive approach that considers clinical presentation, medical history, and, when necessary, laboratory testing. By accurately identifying candidiasis, healthcare providers can initiate appropriate treatment regimens and implement preventive measures to improve patient outcomes and reduce the risk of recurrence. (Nursing Paper Example on Candidiasis)
Treatment Regimens and Patient Education
Effective management of candidiasis involves a combination of pharmacological treatments and patient education aimed at addressing the underlying causes and preventing recurrence. Treatment regimens may vary depending on the severity and location of the infection.
For uncomplicated cases of vaginal candidiasis, over-the-counter antifungal medications such as clotrimazole, miconazole, or tioconazole are often recommended. These medications are available in various formulations, including creams, suppositories, and ointments, and are typically applied intravaginally for several days. Alternatively, oral antifungal agents such as fluconazole may be prescribed for recurrent or severe cases.
In cases of oral thrush, antifungal medications such as nystatin oral suspension or fluconazole lozenges are commonly prescribed. These medications are administered orally and may need to be continued for several days to ensure complete resolution of the infection. In severe cases, systemic antifungal therapy with fluconazole or other azole medications may be necessary.
Cutaneous candidiasis is typically treated with topical antifungal agents such as clotrimazole, miconazole, or ketoconazole creams or powders. These medications should be applied to the affected areas of the skin according to the instructions provided by the healthcare provider.
Invasive candidiasis and systemic infections require prompt initiation of systemic antifungal therapy, usually with echinocandins such as caspofungin, micafungin, or anidulafungin. These medications are administered intravenously and may be continued for several weeks depending on the response to treatment and the presence of underlying risk factors.
In addition to pharmacological treatments, patient education plays a crucial role in the management of candidiasis. Patients should be counseled on proper hygiene practices, including regular bathing, keeping the affected areas clean and dry, and avoiding irritants such as scented soaps and perfumed products. Patients with vaginal candidiasis should be advised to avoid douching and to use cotton underwear and loose-fitting clothing to promote airflow and reduce moisture.
Furthermore, patients with recurrent candidiasis should be evaluated for underlying risk factors such as diabetes mellitus or immunosuppression and managed accordingly. Lifestyle modifications such as maintaining a healthy diet, managing stress, and avoiding unnecessary antibiotic use can also help prevent recurrent infections.
By addressing both pharmacological treatments and patient education, healthcare providers can effectively manage candidiasis and improve patient outcomes while reducing the risk of recurrence. (Nursing Paper Example on Candidiasis)


:max_bytes(150000):strip_icc()/VWH-JessicaOlah-CommonSymptomsofCarbonMonoxidePoisoning-Standard-460087eaa2ad4058af35e4606caabe07.jpg)

:max_bytes(150000):strip_icc()/what-is-botulism-4142153-FINAL-e8e26c264c8e4e55a73f560fdd87c420.gif)