Nursing Paper Example on Understanding Barth Syndrome
Nursing Paper Example on Understanding Barth Syndrome
Causes
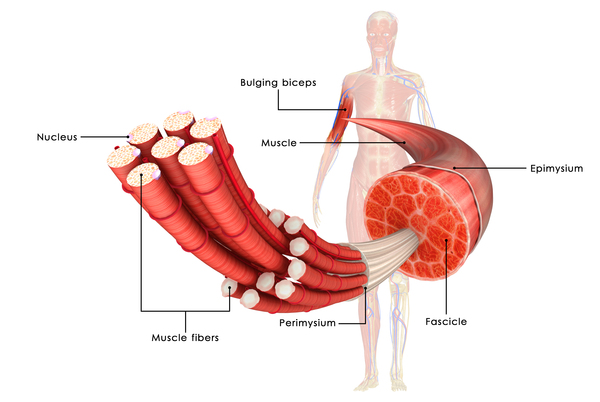
Barth Syndrome arises from mutations in the TAZ gene located on the X chromosome. These mutations disrupt the normal functioning of mitochondria, the cell’s powerhouse responsible for generating energy. The TAZ gene encodes a protein called tafazzin, essential for maintaining the structure and function of mitochondria. When mutations occur in the TAZ gene, tafazzin production is impaired, leading to mitochondrial dysfunction.
The exact mechanism by which TAZ gene mutations affect mitochondrial function is not fully understood. However, it is believed that tafazzin plays a crucial role in remodeling cardiolipin, a phospholipid found in the inner mitochondrial membrane. Cardiolipin is essential for various mitochondrial processes, including oxidative phosphorylation, the process by which cells generate energy.
Mutations in the TAZ gene disrupt cardiolipin remodeling, affecting mitochondrial function and energy production. As a result, cells, particularly those in tissues with high energy demands such as the heart, muscles, and immune system, are unable to function properly. This disruption in cellular energy metabolism contributes to the characteristic symptoms of Barth Syndrome, including cardiomyopathy, muscle weakness, growth delays, and neutropenia.
Since Barth Syndrome is an X-linked genetic disorder, it primarily affects males. Females who carry a single copy of the mutated TAZ gene are typically asymptomatic or may exhibit mild symptoms due to random X-chromosome inactivation.
Barth Syndrome is caused by mutations in the TAZ gene, leading to mitochondrial dysfunction and disruption of cellular energy metabolism. These mutations impair tafazzin production and cardiolipin remodeling, affecting various bodily systems and resulting in the characteristic symptoms of the disorder. (Nursing Paper Example on Understanding Barth Syndrome)
Signs and Symptoms
Barth Syndrome presents a spectrum of symptoms that can vary in severity and may change over time. The hallmark features of Barth Syndrome include cardiomyopathy, muscle weakness, growth delays, and neutropenia.
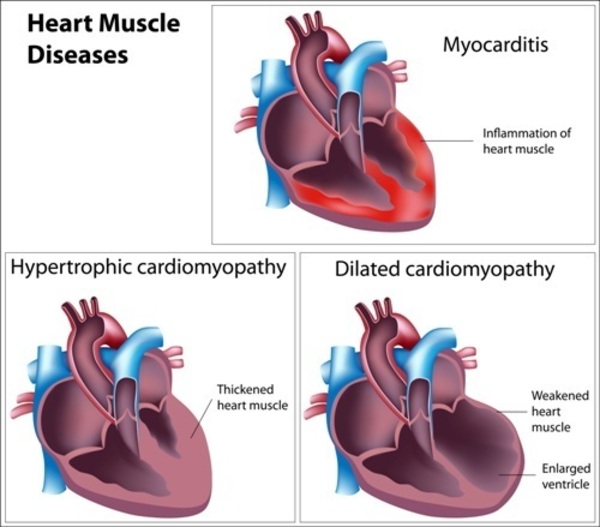
Cardiomyopathy, a condition characterized by weakened heart muscles, is a common manifestation of Barth Syndrome. It can lead to symptoms such as fatigue, shortness of breath, chest pain, and an irregular heartbeat. In severe cases, cardiomyopathy can result in heart failure, a life-threatening condition requiring immediate medical attention.
Muscle weakness is another prominent symptom of Barth Syndrome. Individuals may experience generalized muscle weakness, particularly affecting skeletal muscles used for movement. This can lead to difficulties with mobility, including walking and climbing stairs, as well as challenges with activities of daily living.
Growth delays are frequently observed in individuals with Barth Syndrome, manifesting as below-average height and weight for their age. These growth deficits may become apparent in infancy or early childhood and can persist into adulthood.
Neutropenia, a condition characterized by low levels of neutrophils, a type of white blood cell, is a common hematological feature of Barth Syndrome. Neutrophils play a crucial role in the immune system’s defense against infections. Therefore, individuals with neutropenia are at an increased risk of recurrent bacterial infections, particularly of the skin, respiratory tract, and mucous membranes.
In addition to these hallmark symptoms, individuals with Barth Syndrome may experience other health issues, including feeding difficulties, developmental delays, gastrointestinal problems, and exercise intolerance. The severity and combination of symptoms can vary among affected individuals, making the clinical presentation of Barth Syndrome highly variable. Early recognition and diagnosis of these signs and symptoms are crucial for initiating appropriate medical management and supportive care. (Nursing Paper Example on Understanding Barth Syndrome)
Etiology
The etiology of Barth Syndrome lies in genetic mutations affecting the TAZ gene located on the X chromosome. This gene encodes a protein called tafazzin, which plays a vital role in maintaining the integrity of mitochondrial membranes, particularly through the remodeling of cardiolipin, a phospholipid crucial for mitochondrial function.
The mutations in the TAZ gene result in dysfunctional tafazzin, leading to abnormalities in cardiolipin composition and structure within the inner mitochondrial membrane. This disruption impairs the efficiency of oxidative phosphorylation, the process by which cells generate energy in the form of adenosine triphosphate (ATP). Consequently, affected cells experience energy deficits, leading to the characteristic symptoms of Barth Syndrome.
The inheritance pattern of Barth Syndrome follows an X-linked recessive pattern. Since the TAZ gene is located on the X chromosome, the condition predominantly affects males. Females have two X chromosomes and therefore, if they inherit a mutated TAZ gene on one chromosome, the normal gene on the other chromosome may compensate, resulting in milder or asymptomatic presentation. However, some carrier females may exhibit mild symptoms due to random X-chromosome inactivation.
While most cases of Barth Syndrome are caused by mutations in the TAZ gene, there is significant genetic heterogeneity observed among affected individuals. Variations in the specific mutations and their locations within the TAZ gene can influence the severity and presentation of the disorder. Additionally, other genetic and environmental factors may modulate the clinical features of Barth Syndrome, contributing to its variable expressivity and phenotypic spectrum.
Overall, the etiology of Barth Syndrome is rooted in genetic mutations affecting mitochondrial function, particularly involving the TAZ gene and its role in cardiolipin remodeling. Understanding these underlying genetic mechanisms is essential for accurate diagnosis, genetic counseling, and potential therapeutic interventions for individuals affected by Barth Syndrome. (Nursing Paper Example on Understanding Barth Syndrome)
Pathophysiology
Barth Syndrome’s pathophysiology primarily revolves around mitochondrial dysfunction resulting from mutations in the TAZ gene. Tafazzin, the protein encoded by the TAZ gene, plays a crucial role in cardiolipin remodeling within the inner mitochondrial membrane. Cardiolipin is essential for maintaining mitochondrial structure and function, particularly in oxidative phosphorylation, the process by which cells generate ATP, the energy currency of the cell.
In individuals with Barth Syndrome, mutations in the TAZ gene lead to dysfunctional tafazzin, disrupting cardiolipin remodeling. This disruption affects the stability and fluidity of the mitochondrial membrane, impairing its ability to carry out oxidative phosphorylation efficiently. As a result, affected cells experience energy deficits, leading to various clinical manifestations observed in Barth Syndrome.
Mitochondrial dysfunction in Barth Syndrome primarily affects tissues with high energy demands, such as the heart, skeletal muscles, and immune system. Cardiomyocytes, the cells responsible for heart contraction, are particularly susceptible to mitochondrial dysfunction due to their continuous need for energy to maintain cardiac function. Consequently, individuals with Barth Syndrome often develop cardiomyopathy, characterized by weakened heart muscles and impaired cardiac function.
Skeletal muscles, which rely on oxidative phosphorylation for energy during physical activity, also exhibit dysfunction in Barth Syndrome. This leads to muscle weakness and fatigue, impacting mobility and physical functioning in affected individuals.
Additionally, mitochondrial dysfunction in Barth Syndrome affects the immune system, leading to neutropenia, a condition characterized by low levels of neutrophils, a type of white blood cell crucial for fighting infections. This renders individuals more susceptible to recurrent bacterial infections, further contributing to the clinical manifestations of the disorder.
Overall, the pathophysiology of Barth Syndrome involves mitochondrial dysfunction resulting from mutations in the TAZ gene, leading to energy deficits and cellular dysfunction, particularly in tissues with high energy demands. Understanding these underlying mechanisms is crucial for developing targeted interventions to manage and potentially mitigate the impact of Barth Syndrome on affected individuals.
DMS-5 Diagnosis
Treatment Regimens and Patient Education
The management of Barth Syndrome involves a multidisciplinary approach aimed at addressing the various symptoms and complications associated with the disorder. Treatment regimens focus on symptom management, supportive care, and promoting overall well-being in affected individuals. Patient education plays a crucial role in empowering patients and their families to understand the condition and actively participate in their care.
Medications: Pharmacological interventions are tailored to address specific symptoms and complications of Barth Syndrome. For individuals with cardiomyopathy, medications such as angiotensin-converting enzyme (ACE) inhibitors and beta-blockers may be prescribed to improve cardiac function and manage heart failure symptoms. Other medications may be used to address muscle weakness, growth delays, and neutropenia as needed.
Nutritional Support: Nutritional interventions are essential for individuals with Barth Syndrome to support growth and development, optimize energy levels, and maintain overall health. A balanced diet rich in nutrients, including proteins, carbohydrates, fats, vitamins, and minerals, is recommended. In some cases, dietary supplements or specialized formulas may be prescribed to address specific nutritional deficiencies or challenges.
Physical Therapy: Physical therapy plays a vital role in managing muscle weakness and promoting mobility and functional independence in individuals with Barth Syndrome. Tailored exercise programs, stretching exercises, and muscle-strengthening activities help improve muscle tone, flexibility, and overall physical function. Physical therapists also guide adaptive equipment and assistive devices to enhance mobility and facilitate activities of daily living.
Patient Education: Patient education is essential for individuals with Barth Syndrome and their families to understand the nature of the disorder, its potential complications, and the importance of adherence to treatment regimens. Education sessions provide information on symptom management, medication administration, dietary recommendations, and strategies to optimize overall health and well-being. Patients and families are encouraged to actively participate in treatment decisions, communicate openly with healthcare providers, and seek support from patient advocacy groups and support networks.
Regular Monitoring: Regular follow-up visits with healthcare providers are crucial for monitoring disease progression, assessing treatment efficacy, and addressing any emerging issues or complications. Monitoring may include cardiac evaluations, growth assessments, nutritional status evaluations, blood count monitoring, and other relevant tests as needed.
Overall, a comprehensive treatment approach, coupled with patient education and support, is essential for optimizing outcomes and enhancing the quality of life for individuals living with Barth Syndrome. By addressing symptoms, promoting healthy lifestyle habits, and fostering patient empowerment, healthcare providers can help individuals with Barth Syndrome thrive despite the challenges posed by this complex disorder. (Nursing Paper Example on Understanding Barth Syndrome)











:max_bytes(150000):strip_icc()/VWH-MiraNorian-Encephalitis-4000x2700-d724354b09df4b4a81520987ba5f6b52.png)