Nursing Paper Example on Viral Gastroenteritis
Nursing Paper Example on Viral Gastroenteritis
Stomach flu, or viral gastroenteritis, is an infection causing inflammation in the stomach and intestines, leading to symptoms such as diarrhea, vomiting, nausea, and abdominal pain. While the term “stomach flu” is colloquial, viral gastroenteritis should not be confused with influenza, as it primarily affects the gastrointestinal system, not the respiratory system.
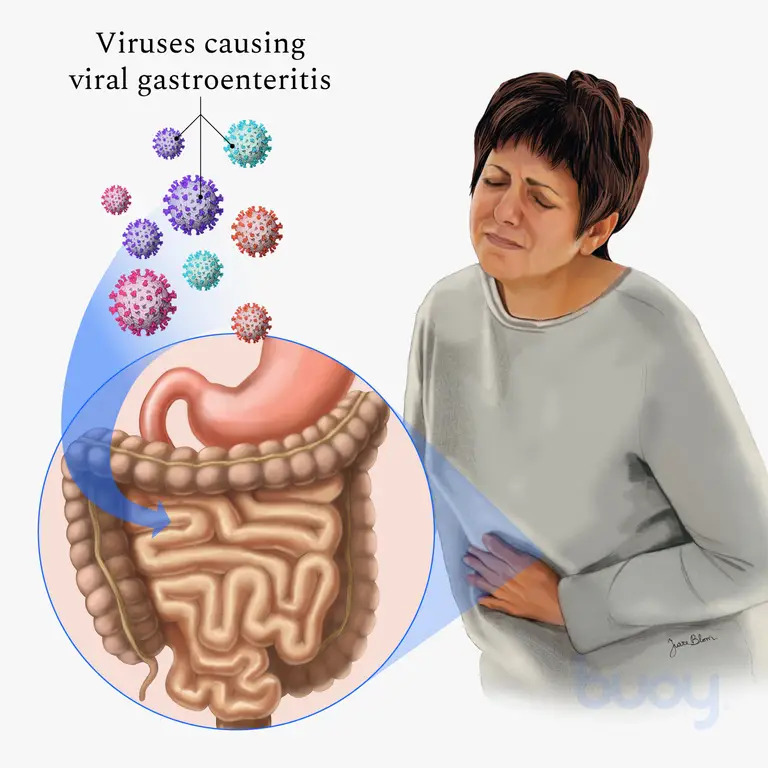
Causes and Pathophysiology
Primary Causes
Viral gastroenteritis is caused by various viruses, including norovirus, rotavirus, astrovirus, and adenovirus. Norovirus is the most common cause among adults and is highly contagious, accounting for the majority of non-bacterial gastroenteritis cases worldwide. Rotavirus is prevalent among children, causing severe diarrhea and leading to high hospitalization rates (Lopman et al., 2016).
Pathophysiological Mechanism
When these viruses enter the gastrointestinal tract, they target and infect the epithelial cells of the intestines. This infection leads to an inflammatory response, disrupting the absorption and secretion processes in the intestines. These disruptions cause rapid fluid loss and electrolyte imbalances, resulting in the hallmark symptoms of viral gastroenteritis. Norovirus and rotavirus are particularly effective at evading the immune system, which allows them to spread rapidly through populations (Karst et al., 2015).
Signs and Symptoms
Initial Symptoms
The onset of viral gastroenteritis is usually sudden, typically beginning with mild fever, chills, and muscle aches. Other symptoms rapidly follow.
Gastrointestinal Symptoms
Common symptoms include watery diarrhea, vomiting, nausea, and abdominal cramps. Dehydration is a major concern, especially in young children and older adults, as frequent vomiting and diarrhea can lead to severe fluid loss. Symptoms generally last between 1 to 3 days but can extend up to 10 days in some cases, particularly for rotavirus in children (Glass et al., 2009).
Systemic Symptoms
In addition to gastrointestinal symptoms, some patients experience headaches, low-grade fever, and body aches. These symptoms tend to be less severe than those associated with respiratory infections.
(Nursing Paper Example on Viral Gastroenteritis)
Diagnosis
Clinical Assessment
Diagnosis is typically based on symptom presentation and medical history. Rapid onset of symptoms like diarrhea, vomiting, and abdominal cramps following exposure to an infected individual or contaminated food is a common indicator. No specific blood tests are required for viral gastroenteritis, but stool samples may be collected in severe or prolonged cases to rule out bacterial infections.
Laboratory Testing
In cases where rapid diagnostic clarification is necessary, laboratory tests such as polymerase chain reaction (PCR) tests or enzyme immunoassays (EIAs) may be conducted to identify the viral strain, especially for outbreaks. PCR testing can detect low viral loads, making it useful for controlling and identifying outbreaks (Lopman et al., 2016).
Transmission and Risk Factors
Modes of Transmission
Viral gastroenteritis spreads primarily through the fecal-oral route, which can occur when individuals consume contaminated food or water or have close contact with an infected person. Norovirus, highly contagious, can spread rapidly through closed environments such as schools, healthcare facilities, and cruise ships. The virus can survive on surfaces for extended periods, increasing its spread potential (Karst et al., 2015).
Risk Factors
The groups at highest risk for severe viral gastroenteritis include young children, elderly individuals, and immunocompromised persons. Poor hygiene practices, inadequate sanitation, and close contact with infected individuals can significantly increase the risk of contracting the illness (Glass et al., 2009).
Treatment and Management
Hydration Therapy
The primary treatment for viral gastroenteritis is supportive care, with oral rehydration solutions (ORS) being essential. ORS contain electrolytes and glucose, promoting water absorption in the intestines, which helps counter dehydration caused by diarrhea and vomiting. Severe cases may require intravenous (IV) fluids to restore fluid and electrolyte balance, especially in vulnerable populations.
Symptom Management
Over-the-counter medications, such as antiemetics for nausea and vomiting, may be used in adults, but are typically avoided in children. Medications like loperamide should be used with caution, as they can worsen symptoms by slowing intestinal motility and potentially prolonging the infection (Kirkwood, 2010).
Nutritional Management
Following initial recovery, patients are advised to consume a bland diet (such as bananas, rice, applesauce, and toast) that is easy on the digestive system. Sugary drinks and caffeine should be avoided as they may aggravate diarrhea and dehydration. Once symptoms improve, patients can gradually reintroduce regular foods.
(Nursing Paper Example on Viral Gastroenteritis)
Prevention
Hygiene and Sanitation
Good hygiene practices are essential to prevent viral gastroenteritis. Regular handwashing with soap and water, especially before eating or preparing food and after using the restroom, is crucial for reducing transmission. Disinfecting surfaces with a bleach-based cleaner helps reduce viral load in environments where an outbreak has occurred (Lopman et al., 2016).
Vaccination
A rotavirus vaccine is available and recommended for infants, which significantly reduces the risk of severe gastroenteritis in young children. Norovirus vaccines are under development but are not yet available for public use (Glass et al., 2009).
Food and Water Safety
Individuals can reduce their risk of viral gastroenteritis by ensuring that food is cooked thoroughly and that water is from a safe source. Avoiding consumption of raw or undercooked shellfish is particularly important, as norovirus is often found in contaminated seafood.
Conclusion
Viral gastroenteritis, or stomach flu, is a common illness characterized by inflammation of the stomach and intestines, resulting in symptoms such as diarrhea, vomiting, and abdominal pain. This condition primarily arises from infections with norovirus, rotavirus, and other viruses, with transmission typically occurring via contaminated food or close contact with infected individuals. While symptoms are usually self-limiting, severe cases, especially in children and elderly individuals, can lead to dehydration and require medical attention. Prevention hinges on proper hygiene practices, vaccination, and safe food handling. As research continues, developing vaccines for more viral strains promises to reduce the global burden of viral gastroenteritis.
References
Glass, R. I., Parashar, U. D., & Estes, M. K. (2009). Norovirus gastroenteritis. New England Journal of Medicine, 361(18), 1776-1785. https://www.nejm.org/doi/full/10.1056/NEJMra0804575
Karst, S. M., Wobus, C. E., Goodfellow, I. G., Green, K. Y., & Virgin, H. W. (2015). Advances in norovirus biology. Cell Host & Microbe, 15(6), 668-680. https://www.cell.com/cell-host-microbe/fulltext/S1931-3128(14)00263-4
Kirkwood, C. D. (2010). Viral gastroenteritis. Infectious Disease Clinics of North America, 24(3), 509-522. https://www.id.theclinics.com/article/S0891-5520(10)00035-1/fulltext
Lopman, B., Zambon, M., & Brown, D. W. G. (2016). The evolving epidemiology of norovirus gastroenteritis: Implications for public health. American Journal of Gastroenterology, 111(1), 21-28. https://journals.lww.com/ajg/fulltext/2016/01000/the_evolving_epidemiology_of_norovirus.6.aspx