Nursing Paper Example on Brucellosis: Understanding Transmission, Clinical Manifestations, and Pathogenic Mechanisms
Causes
Brucellosis, a debilitating zoonotic infection, arises from exposure to various Brucella species, notably Brucella melitensis, Brucella abortus, Brucella suis, and Brucella canis, prevalent in domestic and wild animals worldwide. The primary modes of transmission encompass the ingestion of unpasteurized dairy products, direct contact with infected animals, and inhalation of contaminated aerosols. This bacterial malady poses significant occupational hazards, particularly among farmers, veterinarians, and laboratory workers, due to their close interaction with infected livestock.
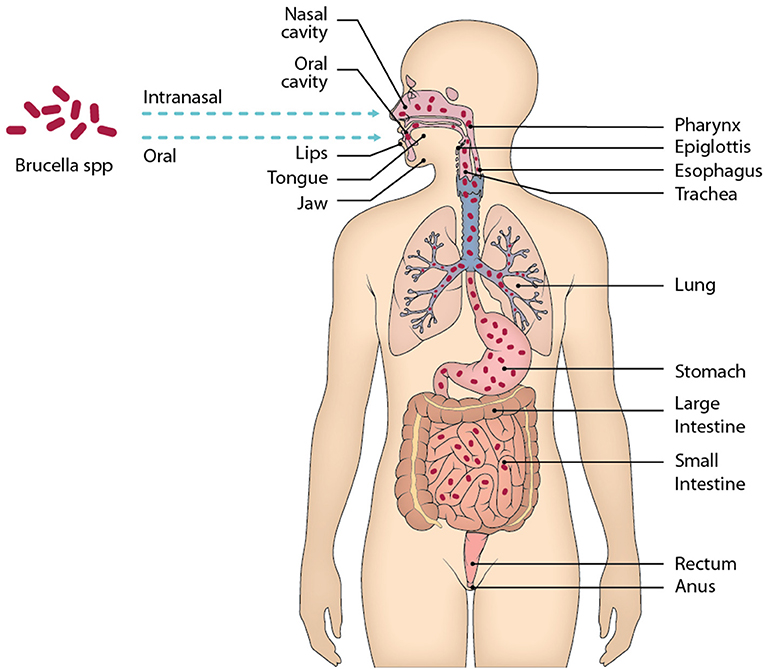
In endemic regions, the consumption of raw dairy products, such as milk and cheese, serves as a major route of transmission. Unpasteurized dairy harbors viable Brucella organisms, facilitating their entry into the human gastrointestinal tract upon ingestion. Additionally, individuals involved in animal husbandry and veterinary practices face elevated risks of brucellosis through direct contact with infected animals, including cattle, goats, sheep, and pigs. Accidental exposure to Brucella-contaminated materials or aerosols in laboratory settings further contributes to the transmission dynamics among healthcare and research personnel.
Furthermore, brucellosis poses a significant threat to hunters, wildlife enthusiasts, and individuals engaged in outdoor activities, as they may encounter infected wildlife, such as deer, elk, and bison, in endemic regions. Inhalation of airborne particles contaminated with Brucella organisms during animal processing or carcass handling amplifies the risk of infection in these populations.
The global distribution of brucellosis underscores its status as a reemerging infectious disease, necessitating heightened vigilance in both agricultural and public health sectors. Addressing the multifaceted transmission dynamics of brucellosis through targeted interventions, including improved food safety measures, enhanced veterinary surveillance, and public health education, is imperative to mitigate its impact on human and animal populations alike. (Nursing Paper Example on Brucellosis: Understanding Transmission, Clinical Manifestations, and Pathogenic Mechanisms)
Signs and Symptoms
Brucellosis manifests as a constellation of nonspecific symptoms, making its diagnosis challenging. The clinical presentation varies widely among affected individuals and may mimic other febrile illnesses, contributing to diagnostic delays. The hallmark symptom of brucellosis is recurrent fever, often characterized by intermittent spikes accompanied by chills and sweats. These febrile episodes may persist for weeks to months, leading to debilitating fatigue and malaise, which can significantly impair daily functioning.
Additionally, individuals with brucellosis commonly experience musculoskeletal symptoms, including arthralgia and myalgia, which may affect multiple joints and muscle groups. The severity of joint pain ranges from mild discomfort to debilitating arthritis, particularly affecting large weight-bearing joints such as the knees and hips. The inflammatory nature of brucellosis-induced arthritis may result in joint stiffness, limited range of motion, and functional impairment, posing significant challenges to mobility and activities of daily living.
Moreover, systemic manifestations such as headache, dizziness, and generalized weakness are frequently reported among brucellosis patients. Profuse sweating, especially during febrile episodes, is a characteristic feature known as “undulant fever,” reflecting the cyclical nature of fever spikes in brucellosis.
Gastrointestinal symptoms, although less common, may manifest as nausea, vomiting, abdominal pain, and diarrhea, mimicking gastrointestinal infections. Neurological complications, such as meningitis and peripheral neuropathy, albeit rare, may occur in severe cases of brucellosis, leading to neurological deficits and cognitive impairment.
Furthermore, brucellosis can result in reproductive complications, including orchitis and epididymo-orchitis in males, and miscarriage or fetal complications in pregnant women. Thus, a comprehensive understanding of the diverse clinical manifestations of brucellosis is essential for timely recognition and prompt initiation of appropriate diagnostic and therapeutic interventions to mitigate its impact on affected individuals. (Nursing Paper Example on Brucellosis: Understanding Transmission, Clinical Manifestations, and Pathogenic Mechanisms)
Etiology
Brucellosis, a debilitating zoonotic infection, is caused by various species of the genus Brucella, gram-negative, facultative intracellular bacteria. Among the several recognized species, Brucella melitensis, Brucella abortus, Brucella suis, and Brucella canis are the predominant etiological agents responsible for human infections. These bacteria primarily infect domestic and wild animals, including cattle, goats, sheep, pigs, and dogs, serving as reservoir hosts and potential sources of human exposure.
Transmission to humans typically occurs through direct contact with infected animals or consumption of contaminated animal products, such as unpasteurized dairy items and undercooked meat. In endemic regions where brucellosis is prevalent among livestock populations, individuals engaged in animal husbandry, veterinary practices, and slaughterhouse operations face heightened occupational risks of acquiring the infection.
Furthermore, brucellosis may be transmitted through inhalation of aerosols containing Brucella organisms during the handling of infected animal tissues or biological specimens in laboratory settings. Healthcare personnel, laboratory workers, and researchers involved in diagnostic and research activities about Brucella species are susceptible to occupational exposure, highlighting the importance of stringent biosafety measures and personal protective equipment (PPE) to prevent infection.
The ability of Brucella species to evade host immune responses and survive within host cells, particularly macrophages, contributes to their persistence and dissemination in the host organism. Upon entry into the bloodstream, Brucella organisms invade and replicate within phagocytic cells, evading intracellular destruction and facilitating their spread to various tissues and organs, including the spleen, liver, bone marrow, and reproductive organs.
Overall, understanding the complex interplay between Brucella species, their animal reservoirs, and human hosts is essential for elucidating the epidemiology and transmission dynamics of brucellosis, guiding the development of effective preventive strategies, and mitigating the burden of this infectious disease on human and animal populations alike. (Nursing Paper Example on Brucellosis: Understanding Transmission, Clinical Manifestations, and Pathogenic Mechanisms)
Pathophysiology
The pathogenesis of brucellosis involves intricate interactions between Brucella species and the host immune system, culminating in chronic and persistent infections characterized by recurrent febrile episodes and systemic manifestations. Upon entry into the host organism, Brucella organisms encounter and invade phagocytic cells, particularly macrophages, employing a myriad of virulence factors to evade host immune detection and subvert intracellular killing mechanisms.
Once internalized, Brucella species establish a specialized intracellular niche within host phagosomes, where they undergo intracellular replication while evading lysosomal fusion and degradation. This intracellular survival strategy enables Brucella organisms to evade host immune surveillance and persist within the host organism for prolonged periods.
The ability of Brucella species to modulate host immune responses and manipulate intracellular signaling pathways plays a crucial role in disease pathogenesis. Brucella-infected macrophages secrete immunomodulatory factors and cytokines, including interleukin-10 (IL-10) and tumor necrosis factor-alpha (TNF-α), which contribute to the establishment of a pro-inflammatory environment and orchestrate systemic immune dysregulation.
Furthermore, Brucella organisms possess a unique lipopolysaccharide (LPS) structure, characterized by a smooth phenotype (S-LPS) or rough phenotype (R-LPS), which influences their interactions with host immune cells and pathogenicity. The S-LPS of Brucella species exhibits immunomodulatory properties, suppressing host immune responses and promoting bacterial survival, whereas the R-LPS, devoid of O-polysaccharide chains, elicits robust inflammatory responses and contributes to the development of protective immunity.
The chronicity of brucellosis is attributed to the ability of Brucella species to establish persistent infections within host tissues and organs, including the spleen, liver, bone marrow, and reproductive organs. This persistent intracellular colonization contributes to the relapsing nature of brucellosis, characterized by intermittent fever spikes and recurrent clinical manifestations, necessitating prolonged antimicrobial therapy and vigilant clinical monitoring to achieve bacterial eradication and prevent disease recurrence. (Nursing Paper Example on Brucellosis: Understanding Transmission, Clinical Manifestations, and Pathogenic Mechanisms)
DSM-5 Diagnosis
Treatment Regimens and Patient Education
Effective management of brucellosis necessitates a multidrug antimicrobial regimen tailored to the individual patient’s clinical presentation, disease severity, and antimicrobial susceptibility profiles of the infecting Brucella species. The cornerstone of treatment involves the administration of combination therapy with antibiotics to ensure optimal bacterial clearance and prevent relapse.
Doxycycline and rifampin represent the preferred first-line regimen for uncomplicated brucellosis cases. Doxycycline, a broad-spectrum tetracycline antibiotic, exhibits excellent intracellular penetration and activity against Brucella organisms, inhibiting protein synthesis within infected host cells. Concurrent administration of rifampin, a potent bactericidal agent, enhances antimicrobial efficacy by targeting bacterial RNA synthesis, thereby preventing the emergence of resistant strains and minimizing treatment failure.
Alternatively, in cases of doxycycline intolerance or contraindications, a combination regimen comprising trimethoprim-sulfamethoxazole (TMP-SMX) and rifampin may be utilized as a second-line option. TMP-SMX, a sulfonamide antibiotic combination, synergistically inhibits bacterial folate synthesis, exerting bactericidal activity against Brucella species when combined with rifampin.
The duration of antimicrobial therapy typically spans 6 to 8 weeks, with prolonged treatment courses warranted in patients with severe manifestations, relapsing disease, or complications such as endocarditis or osteoarticular involvement. Close clinical monitoring and periodic reassessment of treatment response are essential to guide therapeutic adjustments and ensure favorable clinical outcomes.
Patient education plays a pivotal role in brucellosis management by empowering individuals to adopt preventive measures and adhere to prescribed treatment regimens. Key educational components include:
- Food Safety Practices: Emphasize the importance of consuming pasteurized dairy products and avoiding the consumption of raw milk or cheese to prevent brucellosis transmission through contaminated food sources.
- Personal Protective Measures: Educate individuals at high risk of occupational exposure, including farmers, veterinarians, and laboratory workers, about the importance of wearing appropriate personal protective equipment (PPE), such as gloves and masks, when handling potentially infected animals or biological specimens.
- Adherence to Treatment: Stress the significance of strict adherence to prescribed antibiotic regimens and completion of the full treatment course to achieve optimal bacterial eradication and prevent disease relapse.
- Symptom Recognition: Educate patients about the common signs and symptoms of brucellosis, including recurrent fevers, malaise, and joint pain, and encourage prompt medical evaluation if symptoms persist or worsen during or after treatment.
- Follow-Up Care: Emphasize the importance of regular follow-up visits with healthcare providers for clinical monitoring, treatment response assessment, and surveillance of potential treatment-related adverse effects.
By incorporating these educational interventions into comprehensive brucellosis management strategies, healthcare providers can empower patients to actively participate in their care and contribute to the successful prevention and control of this infectious disease. (Nursing Paper Example on Brucellosis: Understanding Transmission, Clinical Manifestations, and Pathogenic Mechanisms)



