Nursing paper Example on Campylobacter Infection
Nursing paper Example on Campylobacter Infection
Causes
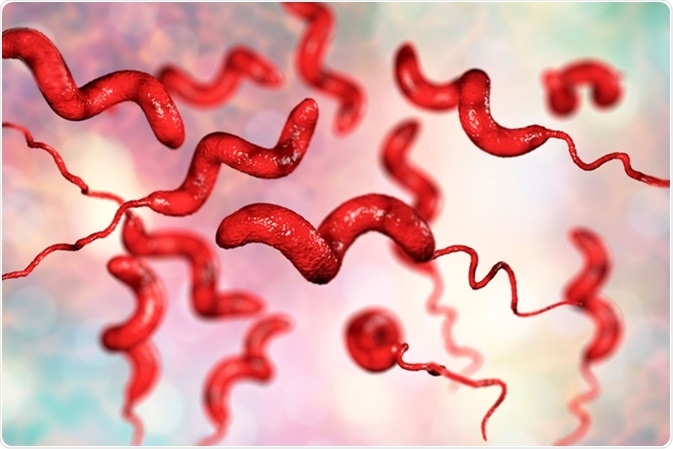
Campylobacteriosis, the most common bacterial cause of diarrheal illness worldwide, is primarily attributed to Campylobacter jejuni and Campylobacter coli, two species within the genus Campylobacter. These gram-negative bacteria are ubiquitous in nature, thriving in diverse environments such as animal intestines, contaminated water sources, and raw or undercooked poultry.
Transmission of Campylobacter species to humans typically occurs through consumption of contaminated food or water. Poultry products, particularly raw or undercooked chicken, are a common source of infection, as Campylobacter bacteria can colonize the intestines of poultry without causing apparent illness. Contaminated water, unpasteurized milk, and untreated recreational water sources also pose significant risks of Campylobacteriosis transmission.
Additionally, direct contact with infected animals or their feces can facilitate transmission of Campylobacter species to humans. Agricultural workers, veterinarians, and individuals engaged in recreational activities involving animals are particularly susceptible to such exposure routes.
The ability of Campylobacter species to survive and persist in the environment further contributes to their transmission dynamics. These bacteria can withstand a wide range of environmental conditions, including low temperatures and exposure to chlorine, enabling their persistence in water sources and food products.
Furthermore, improper food handling and inadequate cooking practices can increase the risk of Campylobacter contamination in food products. Cross-contamination during food preparation, insufficient cooking temperatures, and inadequate hand hygiene practices can all facilitate the transfer of Campylobacter bacteria from contaminated surfaces to food items, thereby increasing the likelihood of infection.
In summary, Campylobacteriosis is primarily caused by the ingestion of contaminated food or water, with poultry products serving as a major reservoir of infection. Understanding the diverse transmission routes and risk factors associated with Campylobacter infection is essential in implementing effective preventive measures and mitigating its impact on public health. (Nursing paper Example on Campylobacter Infection)
Signs and Symptoms
Campylobacter infection manifests with a spectrum of gastrointestinal symptoms, ranging from mild to severe, typically appearing within 2 to 5 days post-exposure. The most common clinical presentation of Campylobacteriosis includes:
- Diarrhea: Diarrhea is the hallmark symptom of Campylobacter infection, often accompanied by loose or watery stools. In some cases, diarrhea may contain blood or mucus, indicating inflammation and tissue damage in the gastrointestinal tract.
- Abdominal Pain: Patients with Campylobacteriosis frequently experience abdominal cramps and discomfort, localized to the lower abdominal region. These symptoms may vary in intensity and duration, depending on the severity of the infection.
- Fever: Fever is a common systemic manifestation of Campylobacter infection, with temperatures often ranging from mild to moderate elevation. Fever typically accompanies other gastrointestinal symptoms and may persist throughout the illness.
- Nausea and Vomiting: Many individuals infected with Campylobacter species report symptoms of nausea and vomiting, which may contribute to dehydration and electrolyte imbalances, especially in severe cases.
- General Malaise: Patients often describe a sense of overall discomfort, fatigue, and malaise, which may accompany the gastrointestinal symptoms. This general feeling of unwellness can significantly impact daily activities and quality of life during the illness.

It is important to note that the severity and duration of symptoms can vary among individuals, with some experiencing mild illness that resolves on its own, while others may develop more severe complications requiring medical intervention. Additionally, certain populations, such as young children, the elderly, and immunocompromised individuals, may be at higher risk of experiencing more severe symptoms and complications associated with Campylobacter infection. Early recognition of these signs and symptoms is crucial for prompt diagnosis and management of Campylobacteriosis, thereby reducing the risk of complications and improving patient outcomes. (Nursing paper Example on Campylobacter Infection)
Etiology
Campylobacteriosis, the infectious disease caused by Campylobacter species, primarily stems from two main culprits: Campylobacter jejuni and Campylobacter coli. These gram-negative bacteria belong to the genus Campylobacter and are renowned for their pathogenicity in humans.
Campylobacter jejuni: Responsible for the majority of Campylobacter infections worldwide, Campylobacter jejuni is a leading cause of bacterial gastroenteritis. It is commonly found in the intestinal tracts of various animals, particularly poultry such as chickens, turkeys, and ducks. Campylobacter jejuni can colonize the intestines of poultry without causing apparent illness, making contaminated poultry products a significant source of human infection.
Campylobacter coli: Although less frequently reported compared to Campylobacter jejuni, Campylobacter coli is another significant contributor to Campylobacteriosis. Similar to Campylobacter jejuni, Campylobacter coli is commonly found in the intestines of animals, including livestock such as pigs and cattle. Consumption of contaminated meat products, particularly pork, has been implicated in Campylobacter coli infections.
Transmission of Campylobacter species to humans primarily occurs through the ingestion of contaminated food or water. In addition to poultry and livestock, other potential sources of Campylobacter contamination include untreated water sources, unpasteurized milk, and cross-contamination during food handling and preparation.
Campylobacter species possess several virulence factors that contribute to their pathogenicity in humans. These include adhesins, which facilitate bacterial attachment to host cells, and toxins that induce inflammation and tissue damage in the gastrointestinal tract. Additionally, Campylobacter species exhibit motility, enabling them to penetrate the intestinal epithelium and evade host immune defenses.
Understanding the etiology of Campylobacteriosis is essential for implementing effective preventive measures and mitigating the spread of infection. By addressing the reservoirs and transmission routes of Campylobacter species, public health efforts can target interventions aimed at reducing the incidence of this common gastrointestinal illness. (Nursing paper Example on Campylobacter Infection)
Pathophysiology
Upon ingestion, Campylobacter species, primarily Campylobacter jejuni and Campylobacter coli, navigate through the gastrointestinal tract, aiming to colonize and establish infection. These gram-negative bacteria possess a myriad of virulence factors that facilitate their pathogenicity in humans.
Adhesion and Invasion: Campylobacter species employ specialized adhesins to adhere to and colonize the intestinal epithelium. These adhesins promote bacterial attachment to host cells, facilitating the establishment of infection. Subsequently, Campylobacter species utilize various mechanisms to invade the intestinal epithelial cells, including secretion of invasion proteins that enable bacterial entry into host cells.
Toxin Production: Once inside the host cells, Campylobacter species produce toxins that contribute to the pathophysiology of infection. These toxins, including cytolethal distending toxin (CDT) and heat-labile enterotoxin (HLT), exert cytotoxic effects on the host cells, leading to cell death and tissue damage. Additionally, Campylobacter species release other virulence factors such as lipooligosaccharides (LOS), which contribute to the inflammatory response in the gastrointestinal tract.
Inflammatory Response: The presence of Campylobacter species triggers a robust inflammatory response in the gastrointestinal tract, characterized by the release of pro-inflammatory cytokines and chemokines. This inflammatory cascade contributes to the clinical manifestations of Campylobacteriosis, including abdominal pain, diarrhea, and fever. Moreover, the inflammatory response can lead to epithelial cell damage, disruption of intestinal barrier function, and increased permeability, further exacerbating the severity of symptoms.
Immune Evasion: Campylobacter species have evolved strategies to evade host immune defenses, enabling their persistence and survival within the host. These include mechanisms to resist phagocytosis by immune cells and modulate host immune responses, allowing the bacteria to establish a chronic infection in some cases.
In summary, the pathophysiology of Campylobacter infection involves a complex interplay between bacterial virulence factors, host immune responses, and tissue damage in the gastrointestinal tract. Understanding these mechanisms is crucial for developing targeted therapeutic interventions and preventive strategies to mitigate the impact of Campylobacteriosis on human health. (Nursing paper Example on Campylobacter Infection)
DMS-5 Diagnosis
Campylobacter infection, or Campylobacteriosis, is a bacterial gastrointestinal illness characterized by specific diagnostic criteria outlined in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). While the DSM-5 primarily focuses on mental health disorders, its criteria can be adapted to aid in the diagnosis of infectious diseases, including Campylobacteriosis.
Diagnostic Criteria:
- Presence of Symptoms: The DSM-5 diagnostic criteria for Campylobacteriosis require the presence of characteristic symptoms, including diarrhea (often bloody), abdominal pain, fever, nausea, vomiting, and general malaise. These symptoms typically manifest within 2 to 5 days post-exposure to Campylobacter species.
- Duration of Symptoms: The duration of symptoms is an essential component of the diagnosis. According to the DSM-5 criteria, Campylobacteriosis symptoms typically persist for up to a week, although the duration may vary among individuals.
- Confirmation of Infection: Laboratory confirmation of Campylobacter infection is crucial for establishing the diagnosis. Stool culture or polymerase chain reaction (PCR) testing is recommended to detect the presence of Campylobacter species in fecal specimens. Positive laboratory results confirm the presence of the bacterium and support the diagnosis of Campylobacteriosis.
- Exclusion of Other Causes: The DSM-5 diagnosis of Campylobacteriosis requires the exclusion of other potential causes of gastrointestinal illness. Differential diagnosis should consider other bacterial, viral, and parasitic infections that present with similar symptoms, such as Salmonella, Shigella, Escherichia coli, and Norovirus.
Clinical Presentation: Clinicians should carefully assess the patient’s clinical presentation and medical history to ascertain the likelihood of Campylobacter infection. A thorough evaluation of symptoms, recent dietary history, and potential exposure to contaminated food or water sources aids in the diagnostic process.
In summary, the DSM-5 diagnosis of Campylobacteriosis involves the presence of characteristic symptoms, confirmation of infection through laboratory testing, and exclusion of other potential causes of gastrointestinal illness. A comprehensive clinical assessment is essential for accurate diagnosis and appropriate management of Campylobacter infection. (Nursing paper Example on Campylobacter Infection)
Treatment Regimens and Patient Education
Management of Campylobacteriosis focuses on alleviating symptoms, preventing dehydration, and reducing the duration of illness through supportive care and, in some cases, antimicrobial therapy. Additionally, patient education plays a pivotal role in preventing further transmission of the infection and promoting optimal recovery.
Supportive Care:
- Hydration: Adequate hydration is paramount in managing Campylobacteriosis, especially in cases of diarrhea and vomiting. Patients are advised to increase fluid intake, including water, oral rehydration solutions, and clear broths, to prevent dehydration and electrolyte imbalances.
- Dietary Modifications: Patients should follow a bland diet that is easy to digest, focusing on bland foods such as bananas, rice, applesauce, and toast (BRAT diet). Avoidance of spicy, fatty, and dairy-rich foods is recommended to minimize gastrointestinal irritation.
- Symptomatic Relief: Over-the-counter medications such as anti-diarrheal agents (e.g., loperamide) and analgesics (e.g., acetaminophen) may provide symptomatic relief from abdominal pain and discomfort. However, caution should be exercised when using these medications, especially in cases of bloody diarrhea or severe illness.
Antimicrobial Therapy:
- Indications: Antimicrobial therapy may be warranted in severe cases of Campylobacteriosis, immunocompromised individuals, or those at risk for complications. Macrolide antibiotics such as azithromycin and erythromycin are the preferred treatment options due to their efficacy against Campylobacter species.
- Duration: The duration of antimicrobial therapy typically ranges from 3 to 5 days, although treatment may be extended in severe cases or immunocompromised patients.
- Consultation: Healthcare providers should evaluate the patient’s clinical status and risk factors before initiating antimicrobial therapy. Consultation with an infectious disease specialist may be warranted in complex cases or cases of antimicrobial resistance.
Patient Education:
- Hygiene Practices: Patients should be educated on proper hand hygiene practices, including frequent handwashing with soap and water, especially after using the restroom and before handling food.
- Food Safety: Emphasize the importance of food safety practices, including thorough cooking of poultry and meat products, avoiding cross-contamination during food preparation, and consuming pasteurized dairy products and treated water.
- Preventing Transmission: Patients should be advised to avoid close contact with individuals who are ill, particularly if they have symptoms of gastrointestinal illness. Additionally, patients should refrain from preparing food for others until symptoms have resolved to prevent further transmission of the infection.
- Follow-up Care: Encourage patients to follow up with their healthcare provider if symptoms persist or worsen, especially if they develop signs of dehydration or other complications.
In conclusion, the management of Campylobacteriosis involves supportive care, including hydration and dietary modifications, and, in some cases, antimicrobial therapy. Patient education on hygiene practices, food safety, and preventing transmission is crucial in promoting recovery and preventing further spread of the infection. By addressing these aspects of care, healthcare providers can optimize patient outcomes and reduce the burden of Campylobacter infection in the community. (Nursing paper Example on Campylobacter Infection)


 Causes
Causes
:max_bytes(150000):strip_icc()/VWH-JessicaOlah-CommonSymptomsofCarbonMonoxidePoisoning-Standard-460087eaa2ad4058af35e4606caabe07.jpg)

:max_bytes(150000):strip_icc()/what-is-botulism-4142153-FINAL-e8e26c264c8e4e55a73f560fdd87c420.gif)